The NJCBM has a world-class neurosciences thrust, working in four cutting-edge areas of research and collaboration across both the central and peripheral nervous systems. We encourage interested scientists to reach out and participate by collaborating or potentially joining this very strong effort.
Neuroscience Initiatives
Peripheral Nerve Regneration (Bioengineering Research Partnership)
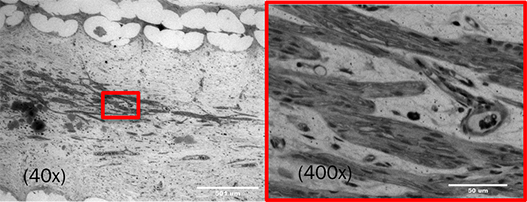
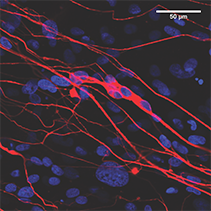
Peripheral nerve injuries require regeneration across gaps to their target muscles. The current standard of care is to use nerve autografts (nerves from elsewhere in the patient’s body), but this involves significant donor site morbidity, mismatched repairs, and increased costs. An alternative to autografts is nerve guidance conduits (NGCs), comprised of three components - a wall, filler, and bioactive molecules - in an infinite number of potential variations and combinations. We believe that the development of effective NGCs is especially beneficial for the unsolved problem of long nerve gaps and gaps across articulating joints.
Funded by the NIH’s National Institute of Neurological Disorders and Stroke, the Bioengineering Research Partnership collaboration combines three technologies invented in three laboratories at Rutgers, toward translation of a device for repairing these large gaps, and specifically with appropriately directed motor-sensory pathways. Neurobiology, biomaterials and bioengineering researchers are conducting laboratory and preclinical studies to create a prototype off-the-shelf NGC for peripheral motor nerves, that enhances not only the rate of regeneration, but also the accuracy of restored motor pathways.

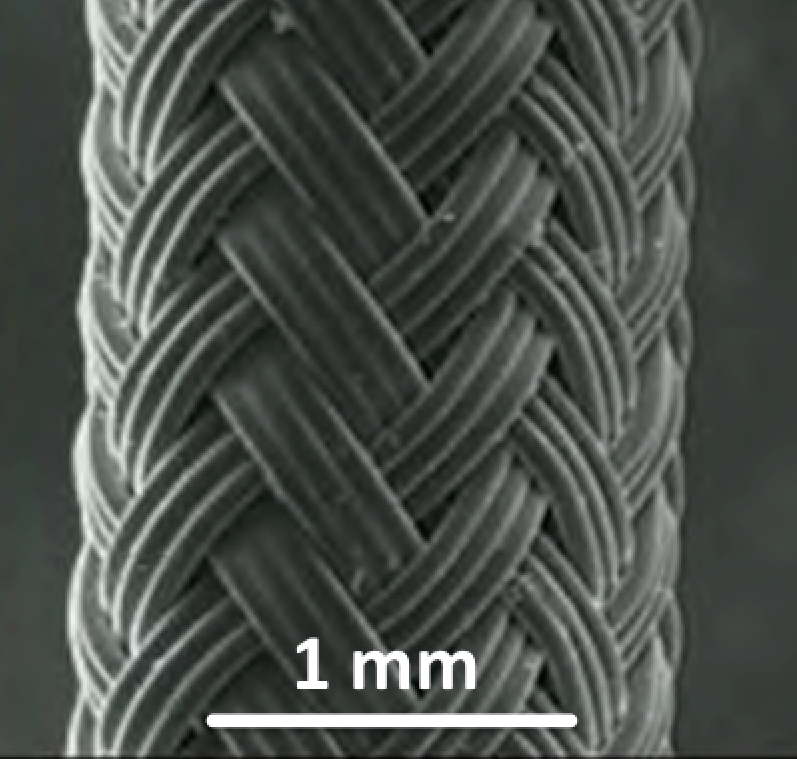
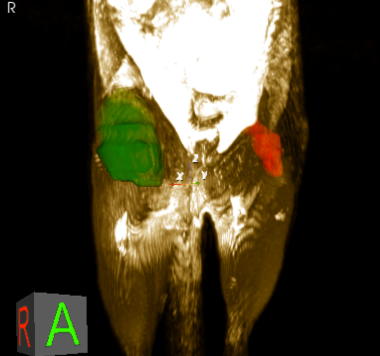


Brain Machine Interfaces (New Jersey Commision on Spinal Cord Research)
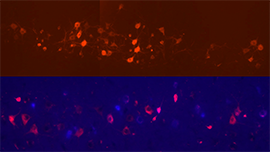
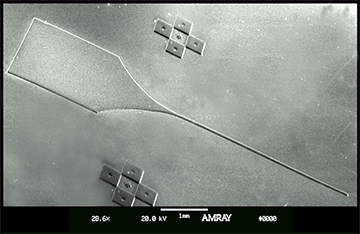
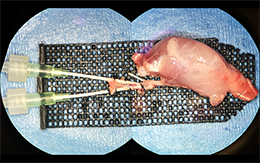
Brain-Machine Interfaces aim to overcome the paralysis and anesthesia of spinal cord injury by integrating motor and sensory brain functions with their peripheral targets (muscles and sensory organelles, respectively). Microelectrodes offer a functionally ideal Brain-Machine Interface for recording and stimulating the cerebral cortex. Due to their rigidity and size, however, they usually only perform adequately for weeks to months, before scar tissue (gliosis) walls them off to leave them clinically useless. This project joins forces between NJCBM’s biomaterials scientists and Rutgers biomedical engineers, to develop novel microelectrodes, called probes, that are so small and flexible that they can reduce gliosis in order to remain functional for years. Implanting such soft and small probes, however, is like trying to push a wet noodle into jello! A novel, tyrosine-derived, degradable polymer coating is therefore used to temporarily stiffen these probes, so that they may be inserted into the brain, before the coating resorbs within hours. We have tested multiple form-factor combinations in vivo, to optimize probe/coating designs; and are currently functionalizing the electrodes for chronic recording studies.

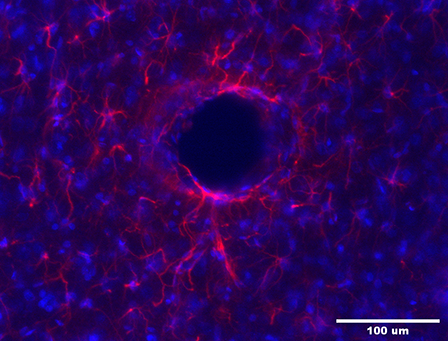

Decellularization & Recellularization of Nerves & Composite Musculoskeletal Tissues (Osteo Science Foundation)
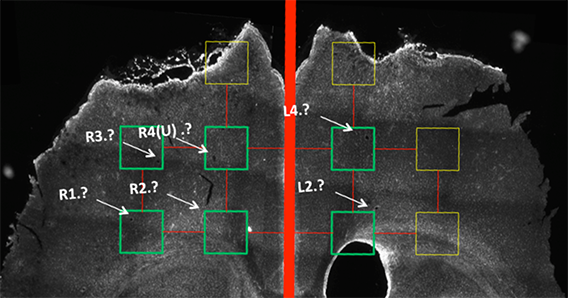
In traumatic craniofacial and musculoskeletal injuries, and massive burn scarring, quality of life is dependent on restoring form and function. Regeneration within the scarred soft tissues and large bony defects is highly dependent on robust vascular supply and sensory-motor reinnervation. This project aims to decellularize donor neurovascular bundles, with and without the muscle groups they supply, so that these tissues can then be re-endothelialized and recellularized with recipient cells, to create autologous (host) tissues for transplantation. This will provide off-the-shelf scaffolds that can readily be made into tissues that are considered “self”, for reconstructing large avascular, asensate and/or paralyzed defects. By providing vascularized nerve grafts of required diameter and length, large peripheral nerve gaps can be repaired; engineered tissues can be innervated and vascularized; and blood supply and sensation can finally be returned to scarred or irradiated tissue beds; all without the risk of any rejection.


RUNEG - Rutgers University Neuro-Engineering Group
RUNEG is a consortium of 17 neuroengineering faculty that was created in March 2014 with the mission of facilitating translational research in the development of devices that enhance central and peripheral nerve regeneration, restoration of motor and sensory function, and transmission of neural signals by brain-computer interfaces. RUNEG was conceived and implemented by Joachim Kohn, Director of NJCBM and Board of Governors Professor of Chemistry, and is currently supported by an award from Chancellor Richard Edwards together with discretionary seed funds from NJCBM.
While focused on biomaterials science and the engineering disciplines, RUNEG brings together researchers from neuroscience, chemical biology, imaging, stem-cell technology, nanotechnology, and computational modeling, as well as physicians. RUNEG fosters these collaborative and interdisciplinary research efforts to enhance understanding of the multidisciplinary field of neural engineering, devices, and cellular therapies to enable the translation of technologies from bench to bedside. The group partners actively with members of the biomedical device and pharmaceutical industries, to accelerate the transfer and commercialization of inventions and technologies into clinically useful products and therapies through a streamlined approach. An important objective of RUNEG is to provide cutting-edge training and education, in the form of seminars and annual workshops, delivered by both world-class investigators within RUNEG and invited leaders in these fields.